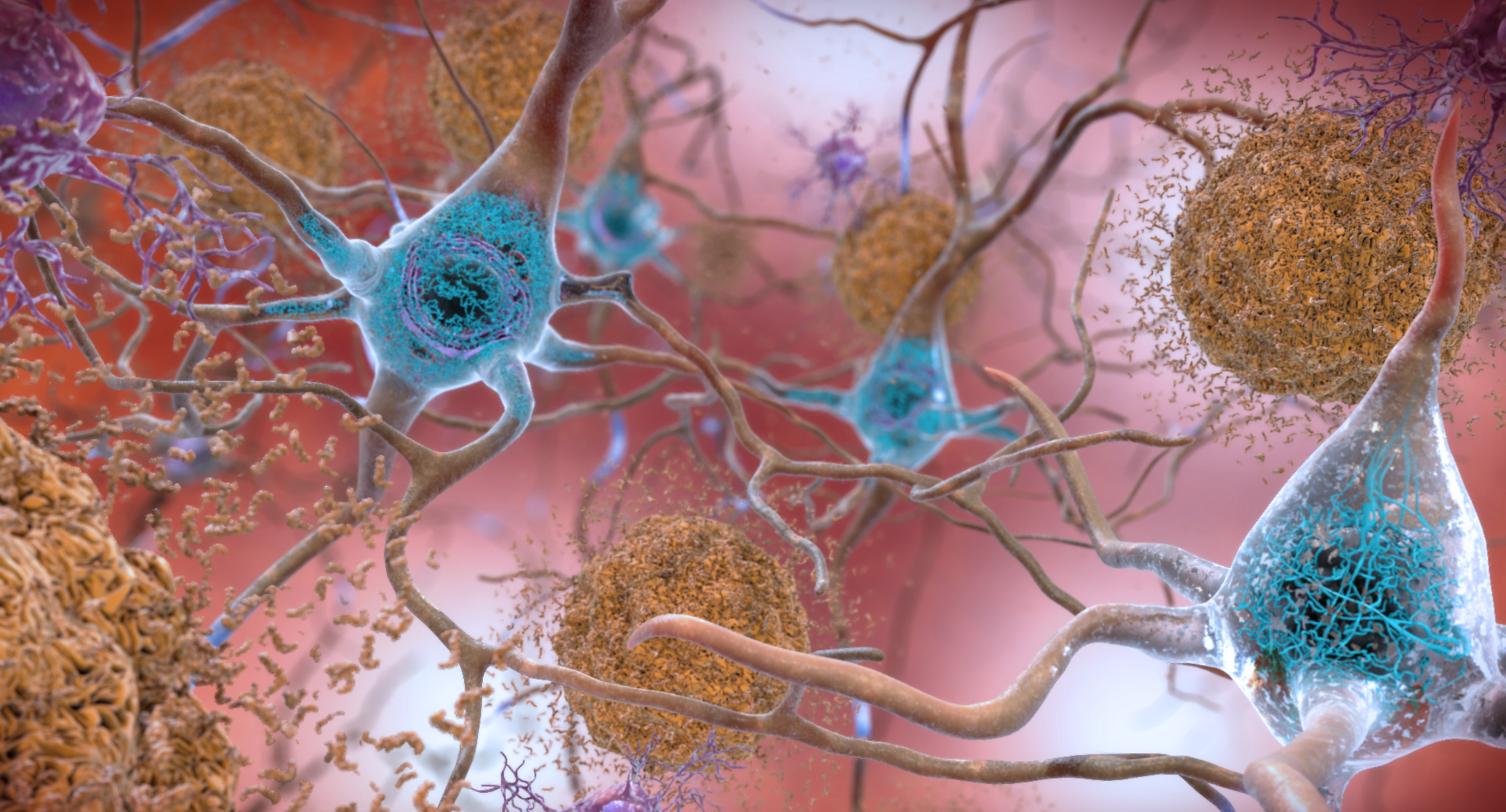
PP2A in Alhzeimers Disease
PP2A activity is suppressed in Alzheimer’s Disease (AD), often by over-expression of endogenous inhibitor proteins such as CIP2A, SET and PME-1. Atux Iskay compounds restore normal PP2A function and ameliorate tauopathy and neuroinflammation associated with progressing AD. We we plan to advance our brain penetrant lead compound ATUX-1215 as an experimental therapeutic for AD with recently awarded Phase 2 SBIR funding.
Beta-amyloid Plaques and Tau in the Brain (Library of Congress)
PP2A and Cancer
Suppression of PP2A activity is a very common, and possibly obligate, event in the transformation of normal to cancerous cells. Atux Iskay PP2A activator compounds restore PP2A function and, in the context cancer, this induces apoptosis (controlled cell death) of tumor cells. Our compounds suppress expression of MYC and MYCN which are known drivers cancer initiation, proliferation and metastasis. Prototype PP2A activator compounds also promote RNAPII stalling in transcriptionally addicted cancers by promoting the formation of non-classical Integrator-PP2A complexes which dephosphorylate the c-terminal domain (CTD) of RNAPII and suppress gene transcription in hyperproliferating cancer cells. This is a newly discovered tumor suppressive mechanism for PP2A.
Ras-Driven Cancer (National Cancer Institute / Univ. of Virginia Cancer Center)
PP2A in Respiratory Diseases
Environmental insults to the lung from, for example, air pollution or smoking frequently suppress PP2A activity. In collaboration with our academic colleagues we have shown that cigarette smoke cleanly up-regulates CIP2A, an endogenous PP2A inhibitor protein, in lung epithelial cells. This has multiple pathological sequelae including up-regulation of cathepsin S, a key proteolytic enzyme, which is responsible for alveolar enlargement, the underlying feature of emphysema, and loss of lung function associated with chronic obstructive pulmonary disease (COPD). A second, less prevalent, though devastating and incurable lung disease is idiopathic pulmonary fibrosis (IPF). IPF is a disease of hyper-proliferating and hyper-activated lung fibroblasts, which secrete excessive amounts of fibrotic extracellular matrix leading to fatal lung scarring. We, and others, have shown that IPF fibroblasts have suppressed PP2A activity. We have recently reported that PP2A signaling can be normalized by treatment with our lead PP2A activator compound ATUX-1215.
Figure by Pngtree
We continue to explore the potential of PP2A modulation in other areas and welcome the opportunity to collaborate with academic investigators.